Medivis Gains FDA Clearance for AR Spine Navigation Platform
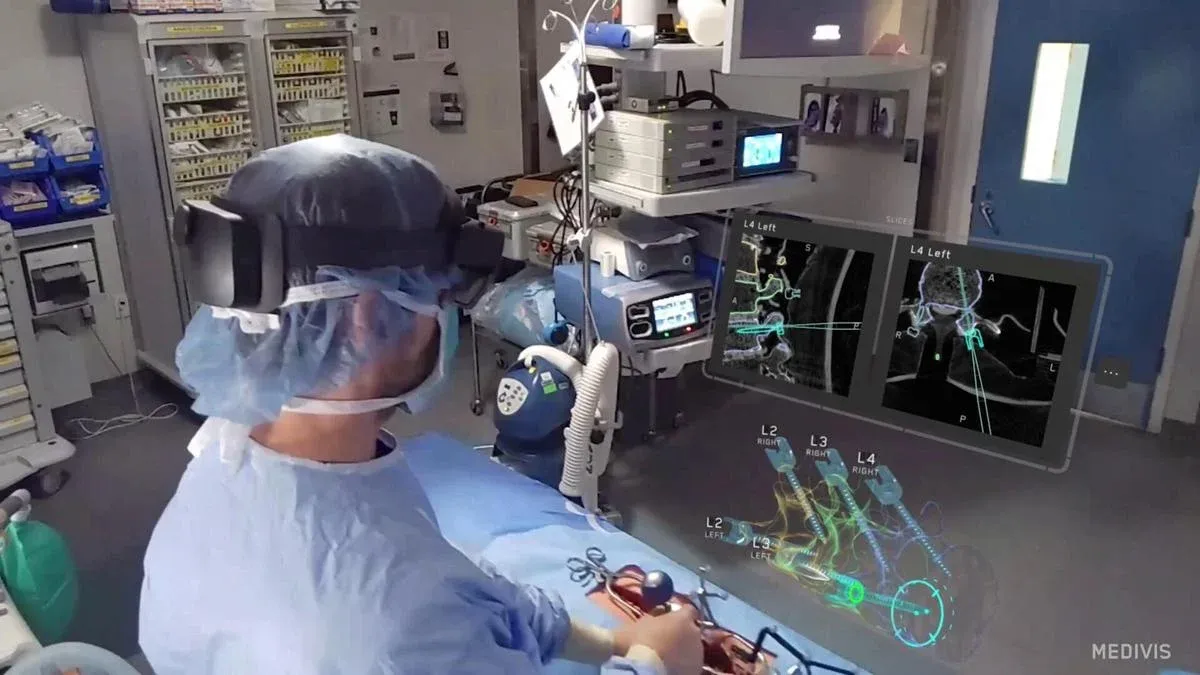
- The FDA has cleared Medivis to distribute its new surgical navigation system for spinal procedures.
- The platform uses AR and AI to guide surgeons during complex spine operations.
Medivis has earned regulatory approval from the U.S. Food and Drug Administration for its Spine Navigation system, paving the way for its rollout across the United States. Hospitals and ambulatory surgical centers can now adopt the platform for use in spine surgeries.
“Surgery is entering a new era of advanced computing that enhances precision, speed, and efficiency in the operating room. This is a pivotal moment in medicine, and we are thrilled to deliver a solution that will elevate patient care to new heights,” said Dr. Christopher Morley, President of Medivis on the company's blog.
The system delivers image-based guidance using a combination of spatial computing and real-time data processing. Applicable across a range of surgical approaches, it supports clinicians with enhanced visual tools and adaptive feedback throughout spine procedures.
Key features include lightweight wearable hardware with voice and gesture controls, AI-powered image analysis, and integration with widely used surgical platforms. The company describes the technology as easily deployable across different clinical settings.
Source: YouTube/Medivis
🌀 Tom's Take:
XR has been reshaping the enterprise for years, and this FDA milestone from Medivis shows its deepening role in healthcare. In the operating room, every second counts, and AR is helping surgeons use each one more effectively when it matters most.
Source: Medivis